Boiling Point Of Vinyl Ketone

It is soluble in water and polar organic solvents.
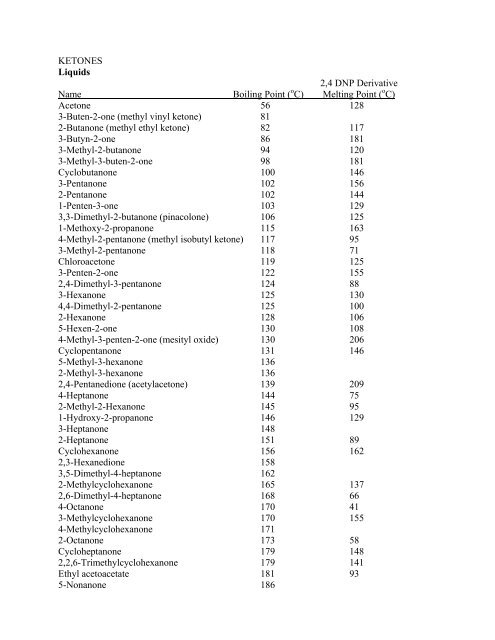
Boiling point of vinyl ketone. Ketones liquids 2 4 dnp derivative name boiling point oc melting point oc acetone 56 128 3 buten 2 one methyl vinyl ketone 81 2 butanone methyl ethyl ketone 82 117 3 butyn 2 one 86 181 3 methyl 2 butanone 94 120 3 methyl 3 buten 2 one 98 181 cyclobutanone 100 146. 1 penten 3 one ethyl vinyl ketone akos009158145. That means that ethanal boils at close to room temperature. Pent 1 en 3 one contains 0 1 bht stabiliser ethyl vinyl ketone 97 stabilized.
Methyl vinyl ketone mvk iupac name. The pharmacokinetics of mek in 70 male and female volunteers following a 4 hr inhalation exposure either alone 200 ppm or in combination with acetone 225 ppm 1 1 25 ratio was described blood and expired air elimination data for the mek exposed individuals revealed on average that mek steady state breath levels of about 10 to 12 ppm were attained non steady state peak blood levels of 3. Industrially it is produced by condensation of acetone and formaldehyde with a subsequent dehydration to give good yields of mvk. Table pageindex 1 shows that the polar single bonds in ethers have little such effect whereas hydrogen bonding between alcohol molecules is even stronger.
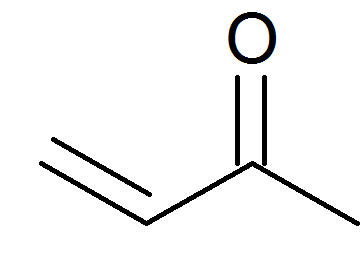
It also dissolves many organic compounds. Scientific opinion of flavouring group evaluation 205 revision 1 fge 205rev1. Butenone is the organic compound with the formula ch 3 c o ch ch 2 it is a reactive compound classified as an enone in fact the simplest example thereof it is a colorless flammable highly toxic liquid with a pungent odor. The size of the boiling point is governed by the strengths of the intermolecular.
Ethyl vinyl ketone 97 stabilized fg. Consideration of genotoxicity data on representatives for 13 a ß unsaturated aliphatic ketones with terminal double bonds and precursors from chemical subgroup 1 2 2 of fge 19. Methanal is a gas boiling point 21 c and ethanal has a boiling point of 21 c. For this reason and because of its low boiling point 56 c 132 8 f which makes it easy to remove by evaporation when no longer wanted it is one.
The other aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger. Methyl vinyl ketone is prepared both on a lab and industrial scale via different routes.